Experienced professional in nickel production
We produce metallic nickel to be used as raw material in the manufacturing of stainless steel and various metal alloys and for surface treatment purposes.
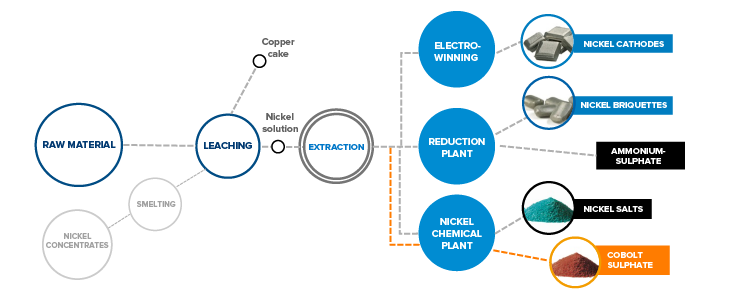
Composed of several hydrometallurgical subprocesses, the production line processes raw nickel matte, nickel sediments and some secondary raw materials.
Read more
LEACHING
Grabs transfer intermediate raw materials from the feeding pit to pulpers from where they proceed to an oxidation leach autoclave as thickener bottom products. After autoclave leaching, the solution is neutralised with calcium in the reactor, filtered and pumped on to the reduction plant for the extraction of calcium. Calcium extraction is followed by the subsequent solution purification phase, cobalt extraction.
Pyrometallurgical raw materials are leached in a stone leaching plant. The solution form of nickel is needed for further refinement. Fine stone is ground with water in the ball mills. Water is then separated, and the ground stone leached using sulphuric acid and oxygen. After leaching in the atmospheric reactor, non-soluble solids are directed into pressure leaching, which takes place at high temperatures in autoclaves. This process dissolves the nickel content of fine stone and allows the extraction of impurities, such as iron and copper. The nickel raw solution is pumped to the extraction department for further purification.
EXTRACTIONS
The nickel raw solution coming from the leaching plant is cleaned from impurities, such as cobalt, at the reduction plant’s extraction and sulphide precipitation departments. The nickel raw solution coming from the leaching plant to cobalt extraction is cooled and washed with kerosene to prevent foreign organic substances from entering the extraction process. Cobalt extraction is carried out in sequential cobalt extraction cells, where the nickel raw solution flows countercurrent to the extraction solution. In addition to cobalt, other impurities, such as copper, lead, zinc and iron, are transferred from the nickel solution into the organic extraction solution during the extraction phase.
The nickel sulphate solution from the leaching plant’s intermediate leaching phase is first directed through calcium extraction, where iron, zinc, copper and manganese are extracted from it, in addition to calcium. After solution purification, some of the purified nickel solution is sent for electrolysis and some for further treatment at the reduction plant. Solution purification separates cobalt from the solution in the form of cobalt raw solution, or cobalt sulphate solution, which is another marketable product.
REDUCTION PLANT
The reduction plant’s end product is nickel briquettes. The purified nickel solution then goes through hydrogen reduction in batches. This is achieved by using hydrogen gas under suitable conditions in the autoclaves. Nickel powder generated in the autoclaves is then separated from the solution by settling and filtering. The nickel powder is dried and then sent on to the powder silo for briquetting or to the powder packaging silo for packaging for customers.
The powder is processed by briquette machines, and the resulting briquettes are sent to either a silo to wait for delivery or for sintering. Completed nitrogen sintered briquettes are packaged for customer delivery. The ammonium sulphate raw solution obtained as the end solution through nickel reduction is sent on to sulphide precipitation for purification. Next, the ammonium sulphate solution obtained from nickel purification is crystallised and dried into a marketable product.
ELECTROLYSIS
The purpose of electrolysis is to produce cathode nickel by means of electrolysis. This is done by means of the electrowinning method, where direct current is fed through a non-soluble lead anode into the electrolyte and onwards to the Ni-cathode.
The Ni-cathode is situated in a diaphragm bag into which the purified nickel solution, or catholyte solution, produced in the leach plant and purified in the extraction phases, is fed. The electrolytic process reduces Ni-ions from the solution onto the cathode surface by means of electricity. The cycle, or growth, of cathodes in the electrolytic vats is approximately seven days. The amount of reduced nickel is dependent on the electric current, current efficiency and the number of vats connected to the circuit. Cathode nickel is cut, packaged and loaded at the cutting plant.
CHEMICAL PLANT
After the extraction phase, nickel sulphate solution goes to the chemical plant for the production of inorganic salts: hydroxycarbonates, hydroxides and sulphates. The department runs several production lines: sulphate, hydroxycarbonate and hydroxide lines.
Sulphate lines produce STD (standard) quality and EN (electroless nickel) quality nickel sulphate crystals. Hydroxycarbonate lines produce so-called dry, paste and granule products. The end products of the hydroxy line are STD and HD quality hyroxide. The sulphate line crystallises the solution through evaporation by means of a continuous process. The crystals are dried, screened, stored in silos and packaged.
The hydroxycarbonate line in continuous operation precipitates the nickel from the solution with soda. The obtained sediment is filtered, washed, dried, stored in silos and packaged. The hydroxide line precipitates hydroxide with lime. The obtained sediment is filtered, washed and dried. Finally the product is stored in silos and packaged.